Sepsis is a life-threatening end-organ dysfunction resulting from dysregulated host responses to infection with physiopathology not fully understood. Yet, it has been long recognized that platelets and neutrophil extracellular traps collaborate to promote secondary tissue damage. Interestingly, patients with hematological malignancies and febrile neutropenia (FN) secondary to chemotherapy, with neutrophils and platelet counts frequently around zero, develop sepsis and septic shock, raising questions about which mechanisms are involved. Here, we show that extracellular vesicles (EVs) and activation of the fibrinolytic system are two potential mediators of tissue damage in sepsis among patients with very low platelets and neutrophil counts.
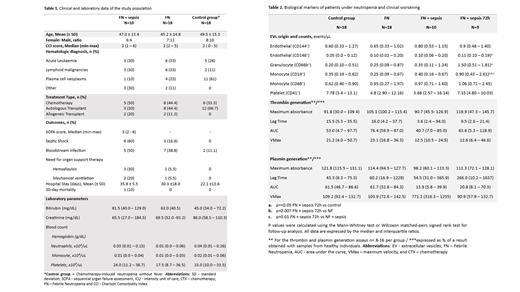
The study population consisted of patients with hematologic malignancies who underwent antineoplastic treatment and that presented neutrophil counts < 500/μl and temperature ≥ 37.5 °C. Sepsis was defined as an increase of at least 2 points in the Sequential Organ Failure Assessment (SOFA) score and septic shock was defined by the need for vasoactive drugs after adequate volume infusion. Samples were obtained after FN diagnosis, at sepsis onset, and 72 h thereafter. A control group of patients with hematological malignancies and chemotherapy-induced neutropenia without fever was also included. Forty-two subjects, both genders, aged from 21 to 71 years were included and categorized into 4 groups, referred to: FN (n=18), FN + sepsis (n=10), FN + sepsis 72h (n=9), and the control group (n=18). The clinical and laboratory characteristics of these groups are described in Table 1.
We first evaluated whether the release of EVs could be associated with the development of sepsis in these patients. EVs were characterized and enumerated from platelet-poor plasma (PPP) obtained from all groups and then separated through high-speed centrifugation (30 min, 20,000 g, 4ºC). EVs were marked with CD146 and 144 for endothelial cells; CD66b for granulocytes; CD14 and CD68 for monocytes, CD41 for platelets, and CD142 for tissue factor (TF) expression. Lactadherin and calcein were used to identify EVs according to size and integrity. Samples were acquired in a flow cytometer, Cytoflex S, with 30 μL/min flow rate and calibration microbeads were used to identify EV of specific sizes (Rosetta). The main findings are shown in Table 2. The main results were an increase in monocyte-derived EVs in patients with FN after sepsis onset (FN + sepsis 72h), compared to all other groups (p=0.01). Moreover, the expression of TF + was higher in these EVs (FN + sepsis 72h) when compared to EVs obtained at sepsis onset (FN + sepsis): 0.50 (IQR 0.29 - 1.10) events/μL versus 0.28 (IQR 0.12 - 0.56) in FN + Sepsis (p=0.03). Additionally, an increase in endothelial (CD146 +) and granulocyte-derived EVs were observed at the same time-point compared to FN and the control group. Of note, monocyte-derived EVs counts were not correlated with monocyte cell counts (Rs= 0.03; p=0.94).
Given that coagulation activation plays a role in the immunothrombotic cascade known to mediate tissue damage in sepsis, we also explored whether thrombin and plasmin generation were increased in these patients. Briefly, the assay used PPP and two independent fluorometric substrates for thrombin (Boc-Val-Pro-Arg-MCA) and plasmin (Boc-Glu-Lys-Lys-MCA) in a 96-well plate, lately initiated by the addition of TF (10 pM). The plate was read in a fluorometric spectrophotometer for 4 h. Results are shown in Table 2, and no differences were observed in thrombin generation. However, the area under the curve of plasmin generation, which could be interpreted as a proxy of the plasma fibrinolytic potential, was lower in both FN + sepsis and FN + sepsis 72 h when compared to FN and the control group.
Our findings reveal that EVs might present a role in the pathogenesis of sepsis in a scenario where cells known to be critical to this process are virtually absent. The increase of TF + EVs from monocytes, which has been demonstrated in classical sepsis, seems to be relevant even in this context, warranting additional studies using functional assays to further explore this mechanism of tissue damage. Our results also demonstrate that hypofibrinolysis is a characteristic of FN-associated sepsis, suggesting that deregulation of the fibrinolytic system is at least in part independent of the participation of granulocytes and platelets in these patients.
Disclosures
Saraiva:Janssen Global Services, LLC: Speakers Bureau; akeda Pharmaceutical Company Limited: Speakers Bureau; AstraZeneca: Speakers Bureau. Costa:Novartis Pharma AG: Honoraria; Pfizer: Consultancy.